The terms used in redox chemistry are very confusing (I've never quite understood them). I'll try to line them all up so they make sense.
Electrons moving along a conductor is what we call electricity, or electric current. It can be used to generate magnetic fields (as in a motor) or heat things up (as in a light bulb), to drive other chemical reactions (e.g. electrolysis or electroplating), or for many other purposes. (What are electrons, anyway?)
History
Until the late 1700s nobody knew there was such a thing as electric current, or how to generate it. Electricity was all the rage, but principally static electricity (surface charges). The discharge of a static charge results in a brief current (lightning, static sparks, "shock" troops and jumping monks), but it cannot be sustained to do useful work.In the 1780s Dr. Luigi Galvani, at the University of Bologna, noticed that muscles could be stimulated to twitch by electric discharges or by contact with dissimilar metals. These studies suggested a connection between electricity and chemistry.
The lead-acid battery was invented in 1859 by Gaston Plante, and the dry cell between 1867 and 1877 by George Leclanché, both of France. The alkaline cell was invented in 1914 by Thomas Edison. Dozens of other types of batteries have been developed since then. They all work by oxidation/reduction chemistry. Here are some pictures of cells of yesteryear.
An electrochemical cell has three main parts: two electrodes and an electrolyte. The electrodes are the dissimilar metals mentioned above. There is an electrolyte that allows ions to move between them. Outside the cell they can be connected by a circuit through which electrons will flow.
A lemon can be used to make a simple cell. The cell has a zinc strip, a copper strip, and the acidic juice of the lemon as the electrolyte. It generates about one volt, but only a very small amount of current. (The voltage of a battery is determined by the materials used as electrodes and electrolyte.)
Lemon Cell
 Here is the site this picture came from, where you can learn how to make a lemon battery.
Here is the site this picture came from, where you can learn how to make a lemon battery.Redox Reactions
Redox reactions happen all the time. Hydrocarbon fuels (such as methane, which was also discovered and isolated by Alessandro Volta) can react with oxygen to be oxidized to carbon dioxide and water. The carbon is oxidized, and gives up electrons, and the oxygen accepts them. Oxidation and reduction are simultaneous. The trick in a battery is to separate the oxidation from the reduction, so the electrons have to go on a trip through the external circuit, where we can make use of them."Oxidation" and "reduction" reactions make a battery work. Oxidation/reduction reactions are electron-transfer reactions. For a battery to work, both an oxidation and a reduction must happen. One generates electrons at one electrode, and the other uses them up at the other electrode. Each of these is called a "half reaction". If the electrodes are connected outside the cell by a circuit, electrons flow and the full reaction is completed.
Oxidation is when electrons are transferred from a substance to oxygen or some other compound. Oxidation doesn't have to involve oxygen, and can be thought of as "de-electronation."
Since electrons are negatively charged particles you can see how this might be related to electricity. Remember that electrons moving along a conductor is electric current. The electrode where oxidation (loss of electrons) takes place is called the anode. On a commercial battery it is marked as the "-" side.
Reduction is when a chemical reactant accepts electrons. It ends up with more electrons than it started with. Reduction could be called "electronation."
Summary of Battery Terms
| Anode | Cathode |
| Oxidation | Reduction |
| De-electronation | Electonation |
| Gives up electrons | Accepts additional electrons |
| - (minus) side | + (plus) side |
The substance that loses electrons is the "reducing agent" or "reductant", while the substance that gains electrons is the "oxidizing agent" or "oxidant". Anions are negative ions moving toward the anode. Cations are positive ions that move toward the cathode.
Summary Illustration
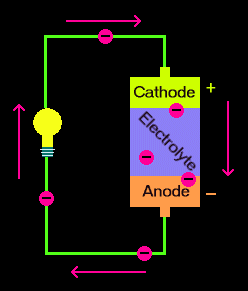
(The picture is from this site.)
OK, So What Makes Electrochemical Cells Work?
Different chemical reactions occur at the anode and the cathode in a cell. The reaction at the anode releases electrons, and leaves behind positively-charged ions. The reaction at the cathode soaks up electrons. Different materials have different tendencies to give up or accept electrons. By choosing the materials for the anode and the cathode carefully, cells with different properties can be designed.
The two dissimilar metals that form the electrodes of a cell have different "reduction potentials". Reduction potential expresses the tendency for a materiel to accept electrons (to be reduced). This reduction potential is expressed in volts. Explanation of "reduction potential". Here is a table of the reduction potentials of various half-reactions.
Here is how the lead-acid battery in a car works:
The anode of each cell is metallic lead, Pb. The cathode is solid lead oxide, PbO2. The electrolyte is sulfuric acid, H2SO4 (which is dissociated into hydrogen (H+) ions and hydrogen sulfate (HSO4-) ions in solution.)
When electrons are allowed to flow (when the battery is under load) this reaction takes place at the anode (- side):
Lead metal is oxidized to bivalent Pb(II), giving up 2 electrons, and reacts with sulfate ion (SO42-) to form lead sulfate and a hydrogen ion:Simultaneously at the cathode (+ side):
Pb + SO4²¯ → PbSO4 + 2e- + H+
The reduction potential of this reaction is +0.356 Volts.
The lead in lead oxide is reduced from tetravalent Pb(IV) to bivalent Pb(II) when the lead oxide reacts with sulfate ions in the electrolyte and hydrogen ions, accepting electrons to form lead sulfate and water:Combined, these two half reactions make up the redox reaction
PbO2 + SO4²¯ + 4 H+ + 2 e- → PbSO4 + H2O
The potential of this reaction is 1.685 volts.
Pb + PbO2 + 2H2SO4 → 2 PbSO4 + 2 H2O
Two electrons were liberated at the anode and flowed through the external circuit to the cathode. The total potential of the reaction is about +2 volts. A 12-volt car battery therefore has six cells in series, each contributing two volts for a total electric potential of 12 volts.This site explains the reactions in a lead-acid cell in more detail.
This site has half-reactions and other information on all kinds of commercial batteries. Highly recommended. Check there to see how a nickel-cadmium, nickel-metal hydride, or alkaline battery works.
major sources:
Powerstream
Electrochem Encyclopedia at Case Western Reserve and
Electrochemical Dictionary at Case Western Reserve
I don't have a link to Volta's original article. Maybe you can find one:
On the Electricity Excited by the Mere Contact of Conducting Substances of Different Kinds, A. Volta, "Philosophical Transactions" Vol. 2, pp 403-431, 1800.good article on Volta and his invention
cool information about early electrical devices
beautiful site summarizing electrochemical cell concepts
David Wheat's Science In Action site has articles about science and math in the real world, weird science, science news, unexpected connections, and other cool science stuff. There is an index of the articles by topic here.
Technorati tags: science, science education, electricity, electrochemistry, batteries, Science In Action

27 comments:
thank you for the info. i really needed it for my science fair project :)
thank you for your awsome information. I needed this for my science project.
Once again thank you!!!!!!
I still don't really get it.
But thanks anyway :)
thanx! i really need this for my science project! :)
thank you it will be useful for my technology project and in the future
thanx for all the info. just like everybody else i needed this fo my science project!! i guess we are all doing the same thing!! well thanx again!!!
well i cant say ill be able to teach it to a group tomarrow but it was helpful....
awesome! huuuuuge help in a huuuuge project due tomorrow! =D
thanks for the info, might help me with my science project! (maybe)
sounnd mert. =) x
Thank you for this. I am trying to make a different type of battery that has a higher specific gravity so it can store more energy for less weight but first i need to know how they work.
Finally!
A blog worth reading on forward until the end! :) I really admire the wording in this blog, quite precise to the details but I just might change one or two things, never-the-less, bravo on well choiced words mate..
I've never known the origin and planning of a battery, quite interesting though I gotta admit.. p.s.>> Thanks for sharing, I actually picked up some knowledge on this one :)
well, like half the other people i'm doing a science fair project about batteries and electricity but your info was sooooooooooo confusing NO OFFENSE! but anyways you should really do something about your facts because they are SOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOO not helpful :(
thanx sooooooo much i had a summary on how batterys work. i was as clueless as a sloth. i really needed the help. although i would change a few things to make it less confusing. hey were all human. and the person before me y dont you critisize bad work. i can tell u r not so bright.thanx again rock on peace out :) :D :P
thanx for this sooooo much i had a hard essay on how the batterie works. although it was a little confusing it was the best site i have been 2. thanx for helpin me big time :) :) :P :p :D :D
Thanks for your info. I really appreciate it.
Thanks so much, this is much more understandable than all the other blogs that I viewed! Some of the information on this blog will be a great addition to my science project! thanks!
Thanks!!!!I actually get it! I needed this for my science project! Very helpful :)
Thanks dude!!!
You got me first prize in a science fair!!!!!
It definitely sheds some light to where from electrons come and go, but I still don't understand how it really works. I mean, it's just some general chemistry explanation. What is " The potential of this reaction is 1.685 volts."?
What is inner battery current path and resistance?
How it all works together?
Daah!
thanks very much, extremely helpful, thanks prof!!!
Dude I do not get what you are saying but thanks!
thanks :D i might need this for sci fair nxt yr and this helped me understand
Thanks for the information im using it for my science fair research
Don't listen to comments from some of the retards here. Coming from an AP Chemistry student, you fairly well explain the higher knowledge of how batteries work. Anyone who doesn't understand this, it is because you haven't learned chemical knowledge; not because our teacher here is bad.
Thank you very much. So good stuff in a quite simple and understandible language. Cox sag ol.
Excellent explanation and very clear. Thanks a lot.
Post a Comment